Different models for atomic structure can be used to explain different phenomena eg. Under the framework of valence bond theory resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure.

The Correct Order Of Hybridisation Of The Central Atom In The Following Species Youtube
NCERT TEXTBOOK QUESTIONS SOLVED.

. Hence 325 which also determines sp3d hybridisation. This can occur in two ways. It is an electron-deficient compound and thus behaves as a Lewis acid.
Given the shapes hybridisation schemes are suggested to describe the bonding in these covalent species. Shape of BF 3 planar. Enter the email address you signed up with and well email you a reset link.
The central roles of the tricarboxylic acid cycle and oxidative phosphorylation in aerobic metabolism will be detailed. Atomic structure in terms of the numbers of protons neutrons and electrons for atoms and ions given the atomic number mass number and any ionic charge. And Ophiodoris niersteinensis sp.
NCERT Solutions Class 11 Chemistry Chemistry Lab Manual Chemistry Sample Papers. Consider the compounds. Find out more about.
Enter the email address you signed up with and well email you a reset link. Isotopes as atoms of the same element with different numbers of neutrons and different masses. Chapter 7 uses the connectivity between the valence shell electron.
Assign the hybridisation of boron in these species. Thus the hybridisation number is 5 which means its hybridisation is sp3d. A Working Method is suggested and illustrated by examples including double bonds and triple bonds in polyatomic molecules.
The new species belong to extant genera and add to the poor. For many chemical species a single Lewis structure consisting of atoms obeying the octet rule possibly bearing formal charges and connected by bonds of positive integer order is sufficient for describing. Another way of finding the hybridisation of a given molecule is with the help of lone pairs and valence electrons.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry including chemical laws diagrams and formulae laboratory tools glassware and equipmentChemistry is a physical science concerned with the composition structure and properties of matter as well as the changes it undergoes during chemical reactions. The pathways used in animals for catabolism and biosynthesis anabolism of some carbohydrates and fat will be covered as well as their control. In BH 4 boron is sp3 hybridized thus the shape is tetrahedral.
According to Kossel and Lewis atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration. Finally how humans adapt their metabolism to survive starvation will be discussed. It features an extensive vocabulary and a.
Ation of the central element. Explain the formation of a chemical bond. The species are based on microfossils extracted from the sieving residues of bulk sediment samples from a flush drill in Nierstein Rhineland-Palatinate.
The Bohr model explains periodic. In BF 3 boron is SP 2 hybridized. Here we describe two new species of ophiuroid Ophiura tankardi sp.
The number of lone pairs in this molecule is 3 and the number of atoms sharing valence electrons is 2. Describe the shapes of BF 3 and BH 4. Nov from the Lower Oligocene of the Mainz Basin.

Aleks Identifying Hybridization In A Small Molecule Youtube
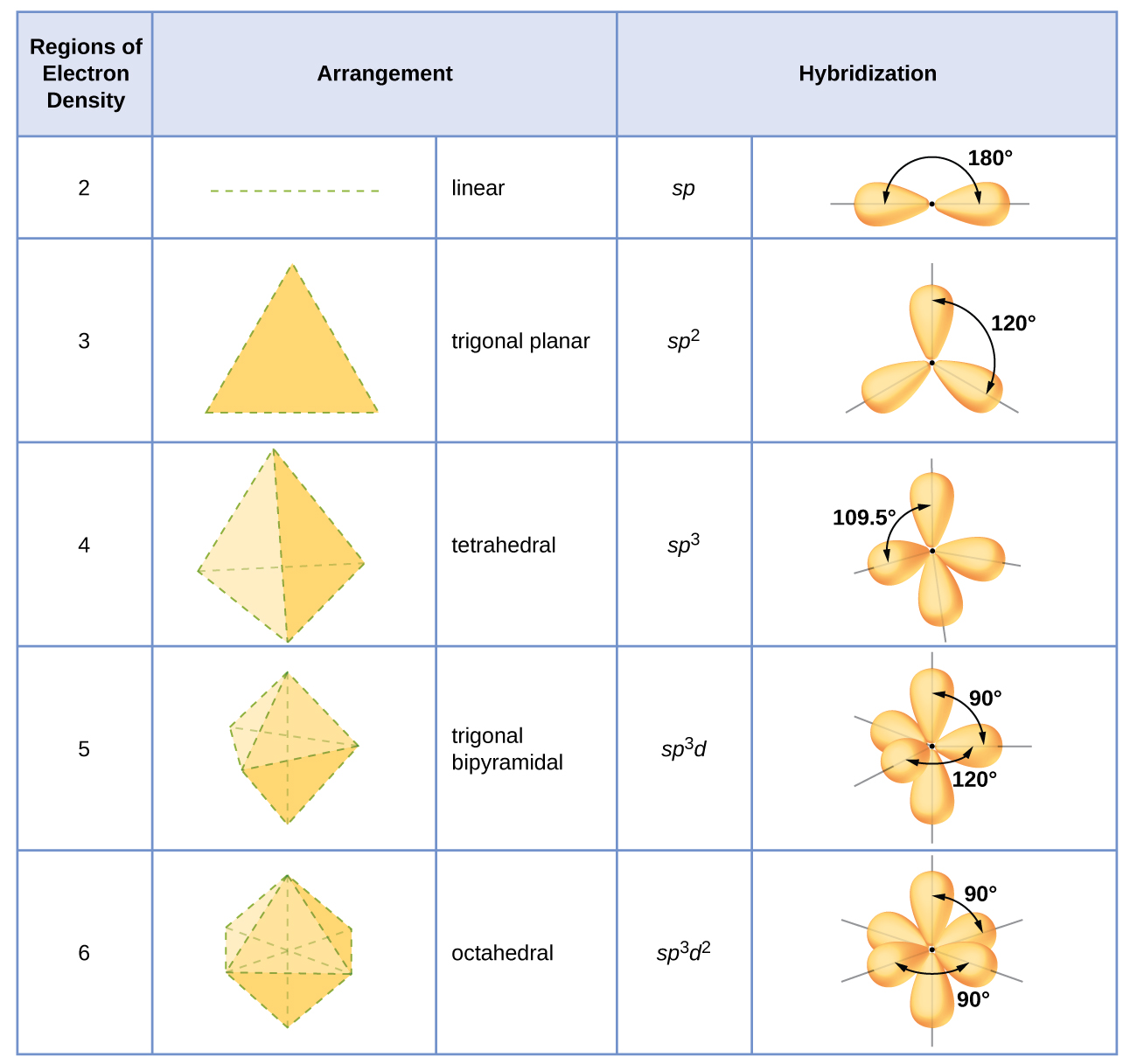
8 2 Hybrid Atomic Orbitals Chemistry

The Hybridization Of The Central Atom Will Change When Youtube
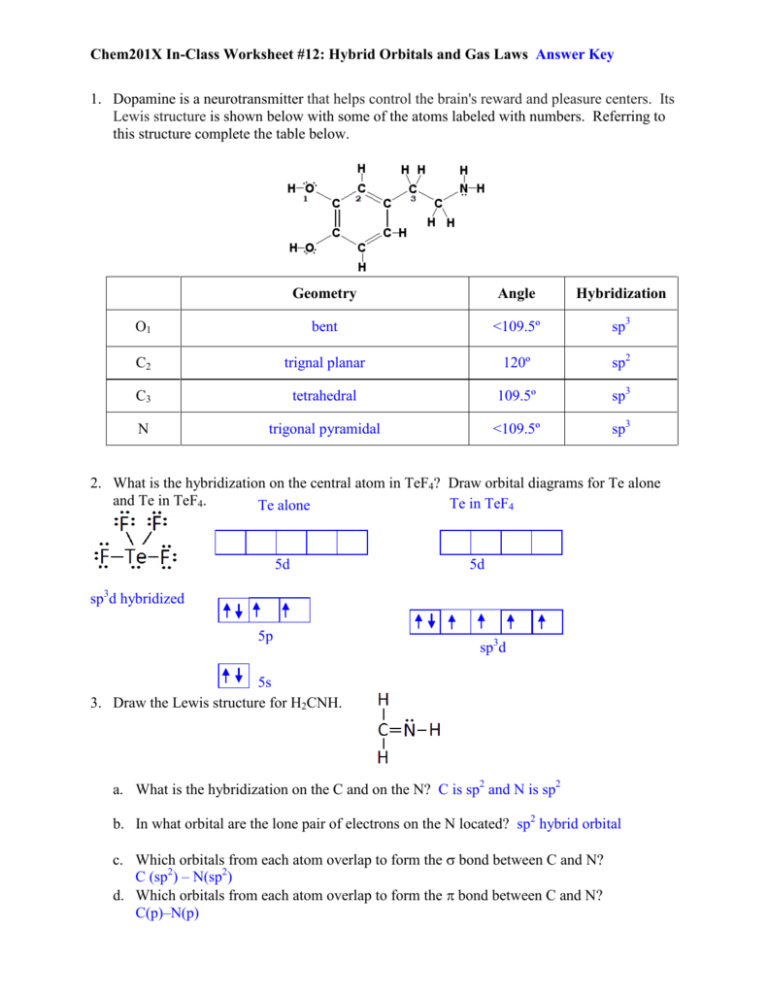
0 Comments